Abstract
Background
Hepatic veno-occlusive disease (VOD), also known as sinusoidal obstruction syndrome (SOS), is an unpredictable, life-threatening complication of conditioning for hematopoietic stem cell transplant (HSCT) and has an estimated overall incidence of 13.7% post-HSCT. VOD/SOS with multi-organ dysfunction (MOD, sometimes referred to as multi-organ failure) may be associated with >80% mortality. In the United States (US), defibrotide is approved for the treatment of adult and pediatric patients with hepatic VOD/SOS and renal or pulmonary dysfunction following HSCT, and it is approved in the European Union for treatment of severe hepatic VOD/SOS after HSCT therapy in patients older than 1 month. Prior to US approval, defibrotide was made available through an expanded-access protocol (T-IND). The present post hoc analysis of final data from the T-IND investigates Day +100 survival in groups of patients receiving autologous or allogeneic transplant.
Methods
Patients received defibrotide 25 mg/kg/day in 4 divided doses, and treatment was recommended for ≥21 days. The original eligibility criteria were VOD/SOS by biopsy or Baltimore criteria (bilirubin ≥2 mg/dL and ≥2 of: hepatomegaly, ascites, ≥5% weight gain) post-HSCT, with MOD (renal and/or pulmonary); the protocol was amended to include also patients after chemotherapy without HSCT (off label), patients without MOD (off label), and patients with VOD/SOS per modified Seattle criteria (≥2 of: bilirubin >2 mg/dL, hepatomegaly, or ≥5% weight gain [in this study]).
Results
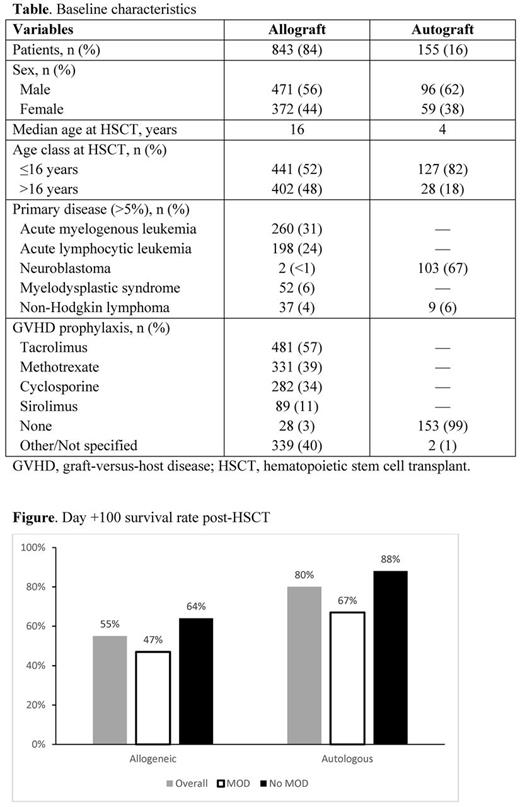
From 2007 to 2016, 1000 post-HSCT patients were enrolled and received defibrotide. Of these, 843 (52% pediatric; 48% adult) patients received allografts (450 of whom had MOD), and 155 (82% pediatric; 18% adult) received autografts (61 had MOD) (Table); 2 had unknown graft types. Acute leukemias were most common in the allograft group, and neuroblastoma was most common in the autograft group. Kaplan-Meier estimated survival rate at Day +100 in the allograft group was 55% (95% confidence interval [CI]: 52%-58%) overall, 47% (95% CI: 43%-52%) for those with MOD, and 64% (95% CI: 59%-69%) for those without MOD (Figure). Kaplan-Meier estimated survival rate at Day +100 in the autograft group was 80% (95% CI: 72%-85%) overall, 67% (95% CI: 53%-77%) for those with MOD, and 88% (95% CI: 80%-93%) for those without MOD (Figure).
Adverse events (AEs) occurred in 74% of allograft patients (in 77% of the MOD subgroup) and 53% of autograft patients (in 64% of the MOD subgroup). Hemorrhage and hypotension were more common post-allograft (31% and 13%, respectively) than post-autograft (17% and 7%). Treatment-related AEs (TRAEs), as determined by investigators, occurred in 21% of allograft patients (22% with MOD) and 20% of autograft patients (30% with MOD); most common (≥2%, either transplant group) were pulmonary hemorrhage (allograft, 5%; autograft, 3%), gastrointestinal hemorrhage (allograft, 3%; autograft, 1%), and epistaxis (allograft, 2%; autograft, 2%). TRAEs leading to discontinuation were more frequent post-allograft (13%) than post-autograft (10%); most common (≥2%) were pulmonary hemorrhage (allograft, 4%; autograft, 3%) and gastrointestinal hemorrhage (allograft, 2%; autograft, 0.6%). TRAEs leading to death occurred in 3% of the allograft group and 0.6% of the autograft group; most common (≥1%) was pulmonary hemorrhage (allograft, 1%; autograft, 0.6%).
Conclusion
In this post hoc analysis of T-IND transplant groups, Day +100 survival rates in both allograft and autograft patients with VOD/SOS, with and without MOD, were consistent with prior defibrotide studies. Taken together, the current results support the overall efficacy and safety profile of defibrotide in patients with VOD/SOS after either allogeneic or autologous HSCT. For both allogeneic and autologous transplant groups, the higher survival rates in the subgroups of patients without vs those with MOD (reinforced by 95% CIs that did not cross) indicate that further study is warranted to determine the impact of treatment earlier in the course of VOD/SOS.
Support: Jazz Pharmaceuticals.
Richardson: Jazz Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Oncopeptides AB: Membership on an entity's Board of Directors or advisory committees. Kernan: Gentium: Other: NAK received grants from Gentium during the conduct of the study, and her research was supported by The National Cancer Institute of the National Institutes of Health under award number P30 CA008748; the content is solely the responsibility of the author . Ryan: Celator/Jazz: Employment, Equity Ownership. Hume: Jazz Pharmaceuticals, Inc.: Employment, Other: stock options. Tappe: Jazz Pharmaceuticals, Inc.: Employment, Other: stock options. Grupp: Adaptimmune: Consultancy; Jazz Pharmaceuticals: Consultancy; University of Pennsylvania: Patents & Royalties; Novartis Pharmaceuticals Corporation: Consultancy, Other: grant.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal